Low-carbon alkanes such as methane, ethane, and propane are abundant on the earth. Transforming these alkanes into higher value-added olefins, aromatics and other chemical products can replace the current chemical production routes that rely on petroleum. The transformation mechanism is of significant application value. Ga/ZSM-5 zeolites exhibit high reactivity in propane aromatization. However, the transformation of propane on Ga/ZSM-5 involves a complex reaction network. Despite of intensive experimental and theoretical studies, the reaction mechanism particularly the aromatization process is still not well understood, which to some extent, hinders the industrial application of this reaction process.
By using in situ ssNMR technology combined with chromatography-mass spectrometry, the research team studied the reaction process of propane aromatization on Ga/ZSM-5. Under batch and flow reaction conditions, the formation and transformation process of the key intermediate cyclopentenyl cations were observed. It has shown that in the batch reaction process the propane aromatization is an autocatalytic reaction, which includes three stages: an initial period, an induction period, and an ending period. The cyclopentenyl cations generated during the reaction can be used as hydrocarbon pool species, which can greatly promote the conversion of propane, thereby accelerating the reaction progress. During the flow reaction process, 12C/13C isotope switching experiments further revealed that cyclopentenyl cations are highly active hydrocarbon pool species that can promote the conversion of propane. Based on the experimental results, the researchers elucidated the reaction mechanism of propane aromatization on the Ga/ZSM-5. Propane is first dehydrogenated on Ga/ZSM-5 to form the initial olefin species at a low reaction rate. The initial olefin will further generate cyclopentenyl cations. In the following process, the cyclopentenyl cations can convert themselves into aromatics and accelerate the dehydrogenation of propane by hydride transfer, which in turn promotes the formation of aromatic hydrocarbons. This research reveals the mechanism of propane aromatization on Ga/ZSM-5 zeolites, which can serve as an important guidance for the industrial application of propane aromatization reactions.
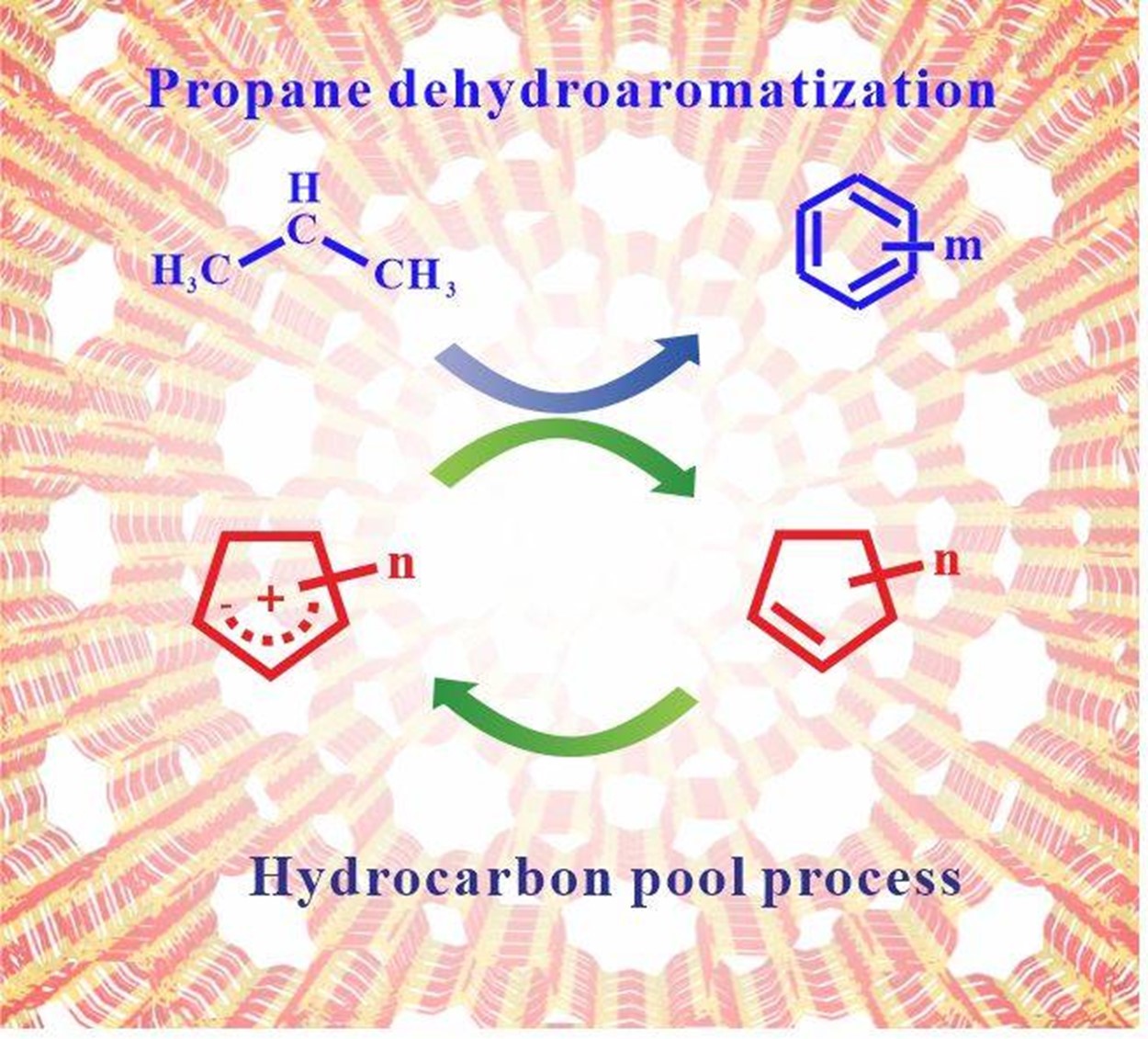
Hydrocarbon pool process in propane aromatization over Ga/ZSM-5 zeolite
Related research results were published in Angewandte Chemie International Edition, titled "Unraveling Hydrocarbon Pool Boosted Propane Aromatization on Gallium/ZSM-5 Zeolite by Solid-State Nuclear Magnetic Resonance Spectroscopy". Associate researcher WANG Chao and Dr. ZHAO Xingling from APM are the co-first authors of the article, and Prof. XU Jun is the corresponding author.
This work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, the Hubei Provincial Department of Science and Technology, and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Full text link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202111111
