A team led by Professor XU Jun and DENG Feng from Innovation Academy for Precision Measurement Science and Technology (APM), CAS in cooperation with Prof. Graham Hutchings group from Cardiff University realized partial oxidation of methane using molecular oxygen on zeolite catalyst. They designed a gold (Au) modified ZSM-5 (Au/ZSM-5) catalyst and achieved the catalytic oxidation of methane to methanol and acetic acid with O2 under mild conditions in aqueous solution. The reaction mechanism was revealed. The work has been published in Nature Catalysis (Latest impact factor: 41.8).
Methane, as the most abundant and cleanest natural carbon resource, exists extensively in natural gas, shale gas, coalbed gas, methane hydrate, etc. Methane is one of the most important C1 building blocks for producing high-value materials and chemicals. Since most of methane is stored in remote areas, transforming methane to transportable oxygenates (methanol, formic acid, acetic acid, etc.) on mining site could improve the efficiency of its utilization. However, its strong C-H bond needs harsh condition (high temperature and pressure) to split hydrogen atom from methane, which is the first step for converting methane to new chemicals. The task just gets trickier by the fact that the desired oxygenates products are often more easily over-oxidized into byproducts (e.g., CO2) than methane. Therefore, the selective methane oxidation is one of the ‘‘holy grail’’ reactions in catalysis. Seeking a route of direct methane oxidation at mild condition with the potential of industrialization has attracted great attention from both academia and industry.
To address this issue, the researchers prepared Au supported ZSM-5 zeolite (Au/ZSM-5) catalyst. Interestingly, this catalyst can achieve methane oxidation using O2 at 120-240 °C in aqueous solution, showing high selectivity to methanol and acetic acid. Quantitative Nuclear Magnetic Resonance (NMR) analysis showed that a maximum oxygenate productivity of 7.3 mol/molAu/h can be obtained at short reaction times. Compared with the Cu-zeolite catalysts on which only C1 products can be produced, the formation of C2 oxygenates as major products on the Au-ZSM-5 catalyst demonstrates a different reaction route. Two dimensional 1H-13C correlation NMR method combined with 13C and 12C isotopes tracing technique were employed to study the detailed reaction mechanism. Au nanoparticles were found to promote the activation of molecular oxygen to surface active oxygen species, which readily reacted with methane. The reaction involves different surface bound intermediates (such as methyl-, methylperoxy- and acetyl-) rather than the species in fluid phase. Importantly, only O2 was employed to produce C2 oxygenates. This is different from the reported noble metals modified zeolite catalysts, such as Rh and Ir, on which necessities CO co-reactant. This work presents a proof-of-concept study on catalytic methane conversions on heterogeneous catalyst using oxygen oxidant. It will enrich our knowledge on this dream reaction.
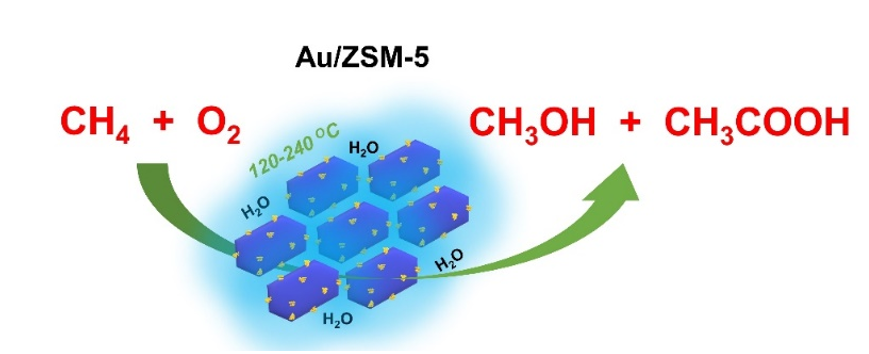
Schematic diagram of the catalytic oxidation of methane to methanol and acetic acid on Au/ZSM-5 (Image by Qi, G., Davies, T.E., Nasrallah, A. et al.)
The result was published in Nature Catalysis, titled “Au/ZSM-5 catalyses the selective oxidation of CH4 to CH3OH and CH3COOH using O2”. Associate Professor QI Guodong from APM is the first author of the airticle. Prof. XU Jun from APM and Prof. Graham Hutchings from Cardiff University are the corresponding authors.
This work is supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences and the Hubei Provincial Department of Science and Technology.
Full text link: https://doi.org/10.1038/s41929-021-00725-8
