On February 28, the research group of LI Conggang and YANG Minghui from the Innovation Academy for Precision Measurement Science and Technology (APM) made new progress in the development and application research of ATP synthase. For the first time, they obtained ATP synthase with a single structural domain, analyzed the molecular mechanism of enzyme catalysis, and applied it to the phosphorylation experiments of various substrate molecules. The related results were published online in the international journal Angewandte Chemie International Edition (Angew. Chem. Int. Ed.).
ATP is the main source of energy in vivo and is vital for life activities. The synthesis of ATP in vivo depends on the ATP synthase protein machinery and the multi-structural domain adenylate kinase (AdK) systems. Microbial fermentation and biocatalytic enzymes, which are commonly used in industrial production, also have drawbacks, such as cumbersome processes and complex operations. Therefore, it is important to develop a simple and efficient ATP synthase with mild reaction conditions for the recyclable energy supply of ATP in vitro and in biological systems.
The histidine kinase HK853 from Thermotoga maritima is an enzyme with multiple catalytic functions, good thermal stability, and substrate specificity. The research team found that its CA structural domain has a new ATP synthesis function. This domain can efficiently synthesize ATP using ADP in a mild condition (Figure 1). Based on this, the research team designed a single structural domain ATP synthase HK853CA, and used nuclear magnetic resonance (NMR) and other advanced technologies to characterize the optimal conditions for HK853CA to catalyze ATP synthesis. The study explored the factors that influence the reaction, such as temperature, concentration, pH, and metal ion binding ability. Through the combination of experiments and molecular dynamics simulations, the molecular mechanism of enzyme catalysis was analyzed. HK853CA can simultaneously bind two ADP molecules to generate ATP and AMP, and the reaction can be reversed under certain conditions (Figure 2). The authors also applied ATP synthase HK853CA to the phosphorylation processes of various substrates (such as proteins, DNA, and small molecules) in biological systems, successfully achieving efficient utilization of ADP and ATP energy molecules (Figure 3).
Based on the molecular mechanism of enzyme catalysis, the research team designed a series of mutations to modulate its catalytic activity. It was found that this catalytic reaction is universal among the histidine kinase family. The results of the study provide a new research direction for subsequent modification and structural optimization of enzymes.
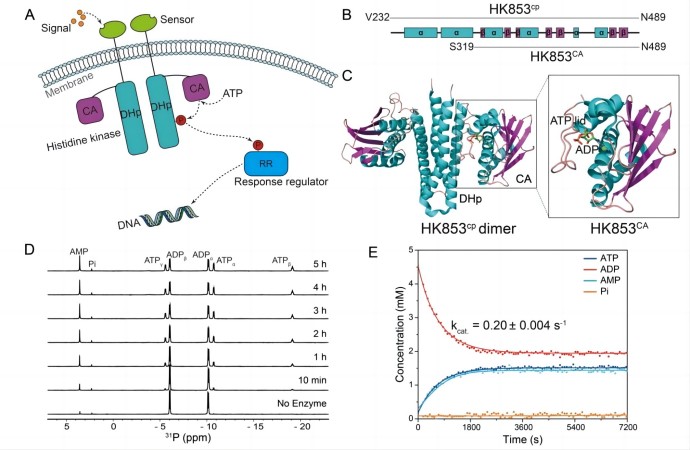
Figure 1. The single-domain ATP synthase designed based on bacterial histidine kinase

Figure 2. The enzymatic catalytic molecular mechanism of the new ATP synthase HK853CA
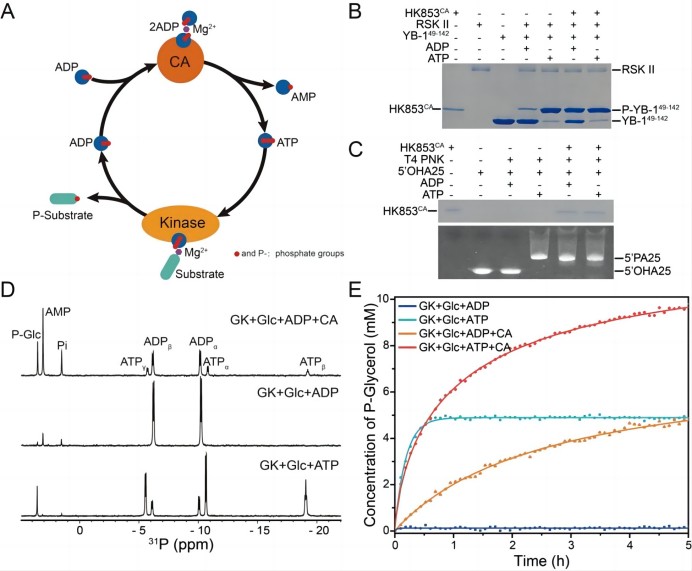
Figure 3. The application of ATP synthase HK853CA in the phosphorylation reactions of proteins, DNA, and small molecules
The related research, titled “An ATP ‘Synthase’ Derived from a Single Structural Domain of Bacterial Histidine Kinase”, was published in the academic journal Angew. Chem. Int. Ed. The first author of the article is JI Shixia, a doctoral student jointly trained by APM of the Chinese Academy of Sciences (CAS) and the Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology. The corresponding authors are associate professor LIU Yixiang and professor JIANG Ling from the In-situ Biomacromolecule Spectroscopy Analysis Group of APM. The molecular dynamics simulation was completed by CHEN Jiawen, an associate professor in the YANG Minghui research group. The research was funded by the National Natural Science Foundation and the Strategic Priority Research Program of the Chinese Academy of Sciences of the Chinese Academy of Sciences.
