Recently, the research team led by DENG Feng and XU Jun from the Innovation Academy for Precision Measurement Science and Technology (APM) made significant progress in the study of the hydrophilicity of carbonium ions in heterogeneous catalytic reactions on molecular sieves. The research found that the cyclopentadienyl carbonium ion formed during the methanol-to-hydrocarbons (MTH) reaction on ZSM-5 molecular sieves exhibits hydrophilicity, which can adsorb water molecules during the reaction and affect its activity, thus playing a regulatory role in the MTH reaction. The relevant results have been published in the Journal of the American Chemical Society.
The MTH reaction is an important chemical reaction that can convert abundant resources such as coal, natural gas, and biomass into essential chemicals and fuels like low-carbon olefins, aromatic hydrocarbons, and gasoline. This reaction is a self-catalytic process, where long-chain olefins and aromatic hydrocarbons formed during the reaction serve as active "hydrocarbon pool" species that catalyze the conversion of reactants into products. Among them, the carbonium ion is a highly reactive species that plays a crucial role as an intermediate in the hydrocarbon pool mechanism. Moreover, water molecules, which are common species in the MTH reaction (produced by methanol dehydration or present in the raw materials), form a localized water environment within the pores of molecular sieves and affect the activity of the "hydrocarbon pool" species. The hydrophilicity or hydrophobicity of the "hydrocarbon pool" species influences the degree of water molecule aggregation near these species, resulting in changes in their activity. Therefore, studying the hydrophilicity or hydrophobicity of the "hydrocarbon pool" species aids in understanding the MTH reaction mechanism and lays a theoretical foundation for optimizing the reaction process.
The research team utilized both magnetic resonance imaging (MRI) and nuclear magnetic resonance spectroscopy (NMR) techniques to investigate the hydrophilicity and hydrophobicity of cyclopentene carbonium ions on ZSM-5 molecular sieves and revealed their role in the MTH reaction mechanism. The study found that during the MTH reaction, the distribution of "hydrocarbon pool" species exhibited a gradient distribution along the reactor length (Figure 1a), with aromatic hydrocarbons mainly concentrated near the inlet and carbonium ions mainly distributed near the outlet. The 1H MRI experiment revealed that water molecules tend to aggregate more readily in regions enriched with carbonium ions (Figure 1b), indicating that carbonium ions, compared to aromatic hydrocarbons, possess hydrophilic properties and are prone to binding with water molecules. Furthermore, the gradient distribution of carbonium ions also results in a corresponding gradient distribution of water molecules within the reactor (Figure 1c). Utilizing two-dimensional 13C-{1H} correlation NMR spectroscopy, the research team discovered that the interaction modes between carbonium ions and aromatic hydrocarbons, as well as water, are entirely different. For carbonium ions, their strong polarity allows them to directly bind with water molecules (Figure 1d). On the other hand, aromatic hydrocarbons exhibit hydrophobic properties, making them less likely to bind with water, and their strong steric hindrance further limits the adsorption of water by the molecular sieve. Reaction performance tests further indicated that the binding of water molecules with carbonium ions facilitates the dehydrogenation aromatization reaction of carbonium ions, leading to the formation of more aromatic hydrocarbon species and subsequently altering the MTH reaction pathway (Figure 1e). This work provides an important theoretical basis for understanding the MTH reaction mechanism and offers effective magnetic resonance spectroscopy and imaging techniques for studying the role of water in complex reaction systems.
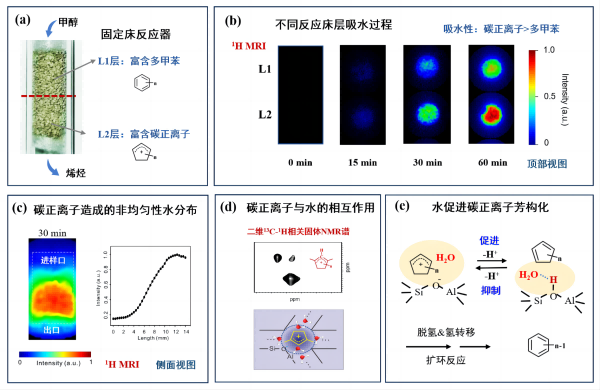
(a) Non-uniform distribution of 'hydrocarbon pool' species in the MTO reactor; (b) Investigating the water absorption process in different reaction beds using 1H MRI technique; (c) 1H MRI spectra showing the non-uniform distribution of water in the reactor; (d) Studying the interaction between carbonium ions and water molecules using two-dimensional 13C-1H correlation NMR spectroscopy;(e) Mechanism of water-promoted aromatization of cyclopentene carbonium ions
The related research titled "Unraveling Spatially Dependent Hydrophilicity and Reactivity of Confined Carbocation Intermediates during Methanol Conversion over ZSM-5 Zeolite" was published in the Journal of the American Chemical Society. Associate Researcher WANG Chao from APM was the first author of the article, while Researcher XU Jun and DENG Feng served as the corresponding contacts for the article.
This research work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Department of Science and Technology of Hubei Province.
Link to the article: https://pubs.acs.org/doi/10.1021/jacs.4c01155
