Recently, the Solid-State Nuclear Magnetic Resonance and Heterogeneous Catalysts Team at the Innovation Academy for Precision Measurement Science and Technology (APM) has made significant progress in the research on the activity and mechanism of photocatalytic selective oxidation of methane. The team discovered that anchoring subnanometric MoOx clusters onto TiO2 nanosheets can effectively suppress the formation of CO2 during the methane oxidation process, significantly enhancing the selectivity for oxygenated organic products. The relevant research findings were published in Nature Communications.
Methane (CH4) is an abundant natural resource, but its chemical inertness poses significant challenges in directly converting it into high-value-added oxygenated compounds (such as methanol, formaldehyde, etc.). Traditional thermocatalytic methods require high-temperature and high-pressure conditions, which not only result in high energy consumption but also exhibit poor selectivity, easily leading to over-oxidation. Photocatalytic technology harnesses solar energy to drive methane oxidation reactions, offering green and economic advantages. However, improving the selectivity and activity of photocatalytic reactions remains an urgent issue to be addressed. The addition of cocatalysts can significantly enhance the separation efficiency of photogenerated charge carriers, thereby substantially improving the activity of methane conversion. However, due to the high cost of conventionally used noble metal (e.g., Au) cocatalysts, the use of inexpensive non-noble metal (e.g., Ni, Co) promoters has attracted widespread attention. Nevertheless, the challenge of simultaneously achieving both high selectivity and conversion rate still persists.
The research team developed a TiO2-based photocatalyst (Figure 1a). By anchoring sub-nanometer-sized (0.6 nm) MoOx clusters on its surface (Figure 1b), they achieved an efficient photocatalytic oxidation reaction of methane. The catalyst with a 0.5% MoOx loading exhibited the optimal catalytic activity, achieving an organic oxygenate yield of 3.8 mmol/g within 2 hours, with selectivity approaching nearly 100% (Figure 1c). The apparent quantum yield at 365 nm reached 13.3%. This catalyst demonstrated an almost constant product formation rate and high selectivity (>95%) throughout the 1800-minute reaction process, exhibiting excellent stability (Figure 1d). Through in situ EPR and NMR spectroscopic studies on the reaction mechanism, it was discovered that MoOx clusters can activate O2 to form surface-active species (Mo−OO and Mo−OOH), which facilitate the activation of methane's carbon-hydrogen bonds to form reaction intermediates (Mo-CH2, Figure 2b). This effectively inhibits the formation of hydroxyl radicals (•OH) and superoxide radicals (O2•−), thereby reducing the over-oxidation of products (Figure 2a). Moreover, the Mo species reduced by photogenerated electrons, in the presence of water, can promote the desorption of formaldehyde from the catalyst surface, thus inhibiting further oxidation (Figure 2c). This study provides new insights for designing efficient non-noble metal catalysts, advancing the practical application of methane conversion technology.
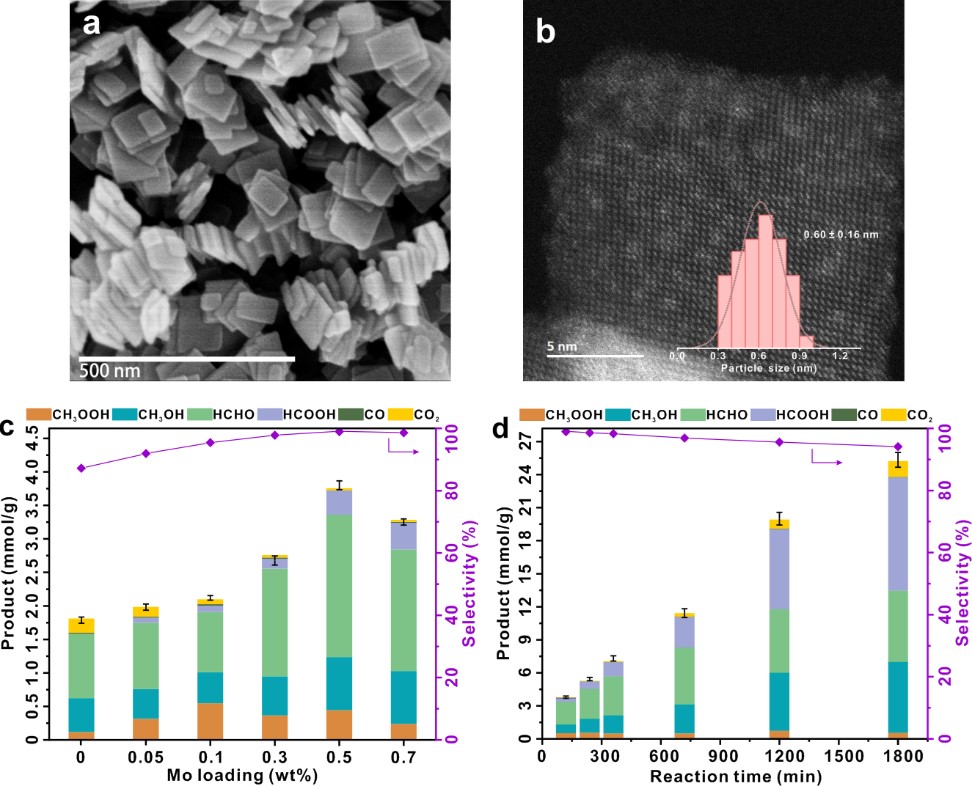
(a) SEM image of TiO2;
(b) ACHAADF-STEM image of 0.5MoOx-TiO2;
(c) Photocatalytic activity and selectivity for methane oxidation over catalysts with different Mo loadings;
(d) Photocatalytic activity and selectivity for methane oxidation over the 0.5MoOx-TiO2 catalyst with extended reaction time.
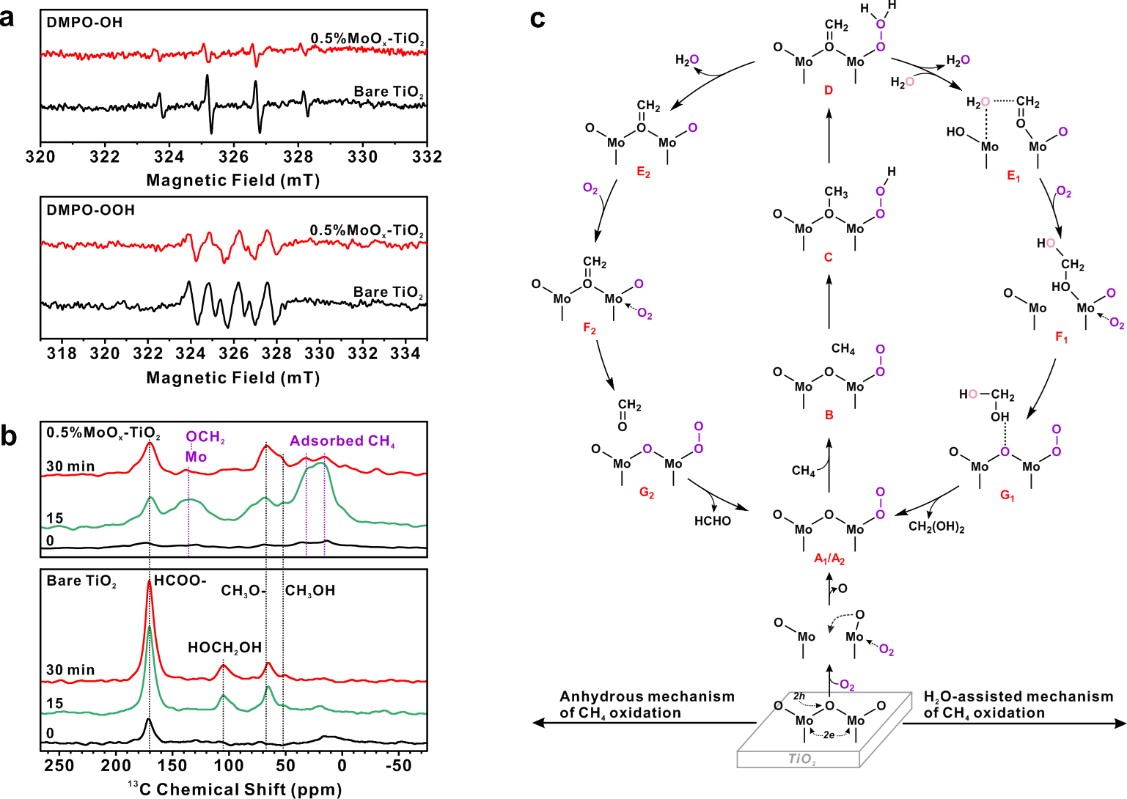
(a) EPR experiment for radical trapping, with DMPO as the trapping agent;
(b) In-situ solid-state NMR experiments on 0.5MoOx-TiO2 and TiO2 catalysts under different illumination times;
(c) Reaction mechanism diagram
The relevant research was published in Nature Communications under the title "Subnanometric MoOx clusters limit overoxidation during photocatalytic CH4 conversion to oxygenates over TiO2". WU Panpan, a doctoral student from APM, is the first author. CHU Yueying, a researcher from APM, and WANG Maolin, a doctoral student from MA Ding's research group at Peking University, are co-first authors. FENG Ningdong and DENG Feng, both researchers from APM, and YE Jinhua, a professor from the National Institute for Materials Science (NIMS) in Japan (the collaborating institution), are corresponding authors.
This research work was supported by the National Natural Science Foundation of China (NSFC) and the Chinese Academy of Sciences (CAS).
Link to the article: https://doi.org/10.1038/s41467-025-59465-z
