Researchers at the Innovation Academy for Precision Measurement Science and Technology (APM) have made a key discovery about zeolites, critical materials in catalysis. Their recent study found that water can activate previously "NMR-invisible" aluminum in ultra-stable Y (USY) zeolite, creating synergistic active sites. This significantly boosts the zeolite's catalytic performance, particularly in converting diethyl ether to ethylene. The findings were published in the Journal of the American Chemical Society.
Zeolite, with ordered microporous structures and tunable acidity, exhibit excellent shape selectivity, activity, and stability in catalytic reactions, thus finding widespread applications in petrochemical and fine chemical industries. Water plays a pivotal role in the zeolite-catalyzed reactions, serving as a solvent, reactant, product, and accelerant. Moreover, it can significantly influence the zeolitic acidity, thereby impacting catalytic performance. The “NMR-invisible” Al, tri-coordinated framework Al and extra-framework Al, are prevalent in the zeolites, considering as the key Lewis acid sites (LAS). However, the dynamic interaction of water and these elusive Al species remain poorly understood, with their catalytic contributions are often overlooked.
To address this knowledge gap, the team conducted a comprehensive investigation into the dynamic interactions between “NMR-invisible” Al species and water in dehydrated USY zeolites by employing advanced solid-state NMR techniques combined with theoretical calculations. The results demonstrate that water undergoes dissociative adsorption on “NMR-invisible” Al sites, leading to a substantial increase of over 60% in Brønsted acid sites (BAS), as well as the formation of Brønsted/Lewis synergistic acid sites. This transformation dramatically enhances the catalytic activity of USY zeolite in the conversion of diethyl ether to ethylene. The evolution of Al species induced by water adsorption was monitored using one-dimensional (1D) 27Al MAS NMR experiments (Figure 1a–b). The water dissociation adsorption transforms “NMR-invisible” Al species into detectable forms, and facilitates the generation of BAS on tetra-, penta-, and hexa-coordinated Al. Furthermore, two-dimensional (2D) 1H–1H DQ–SQ NMR experiments (Figures 2a–e) were performed to investigate the spatial proximity of hydrogen species in the USY samples upon water adsorption. These results (Figure 2f) confirm the formation of Brønsted/Lewis synergistic acid sites, where newly formed Brønsted acid protons are in close proximity to Al–OH groups. Based on these findings, the team successfully elucidated the interaction mechanism between water and “NMR-invisible” Al species. They propose a water-driven regulatory mechanism for modifying zeolite active sites, offering new insights into the catalytic behavior of zeolite in the presence of moisture.
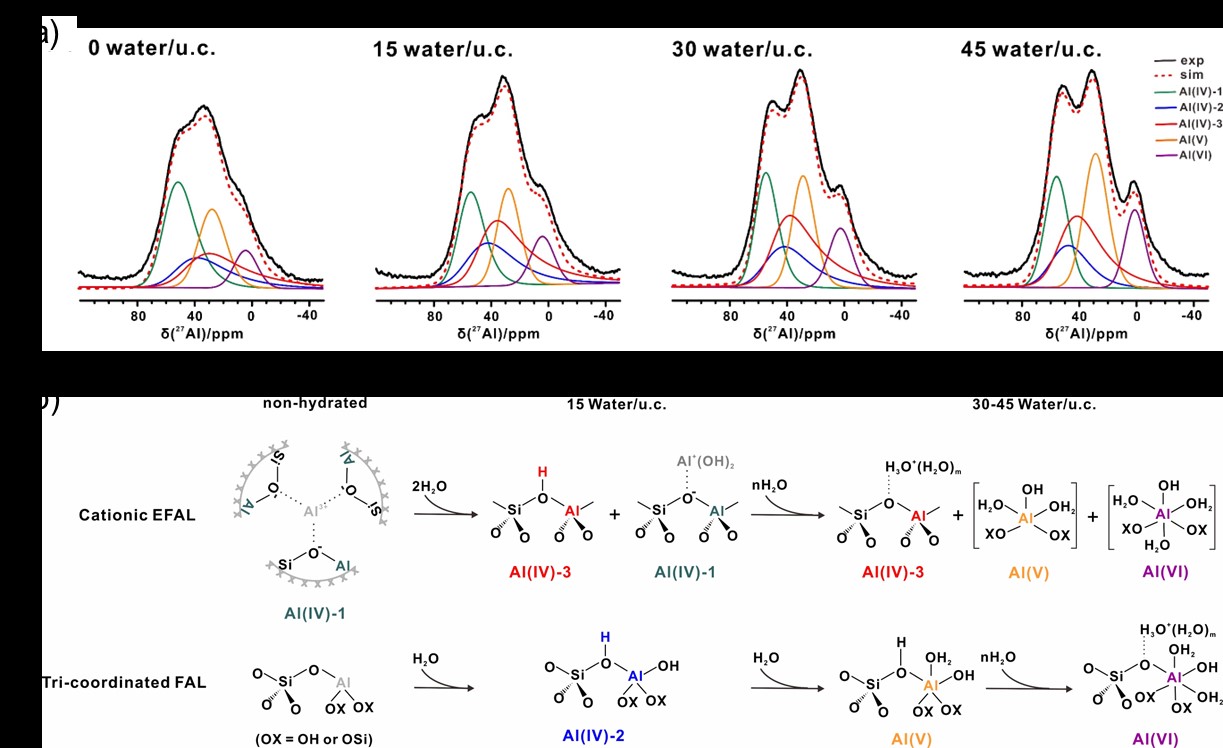
(a) 27Al MAS NMR spectra of USY zeolites with different water loadings (0, 15, 30, and 45 water molecules per unit cell), and (b) schematic of proposed hydration paths involving extra-framework Al cations and tri-coordinated framework Al.
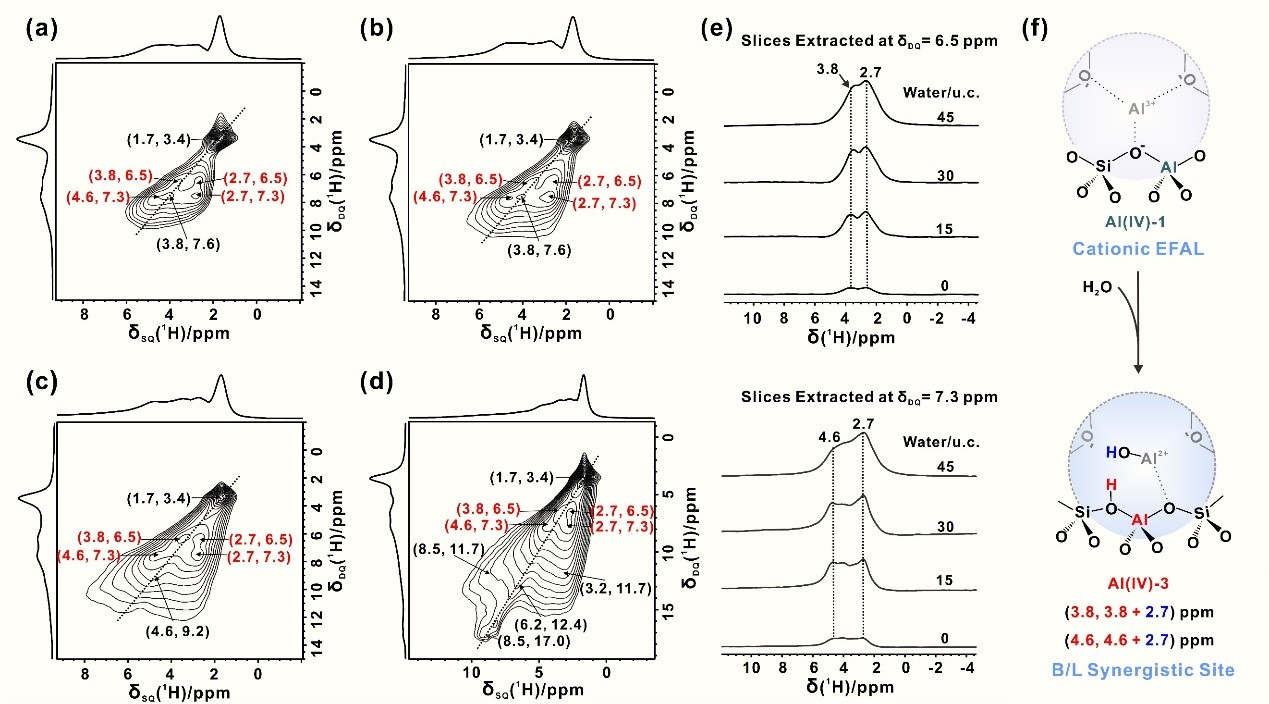
2D 1H−1H DQ-SQ NMR spectra of water adsorbed on USY zeolites with various water loadings: 0 (a), 15 (b), 30 (c), and 45 water/u.c. (d), slices extracted at δDQ = 6.5 and 7.3 ppm from 2D plots (e) and schematic of B/L synergistic sites formation by water hydrolysis on LAS (Al-OH) (f).
The lead author of the paper is WANG Xingxing, a Ph.D. student co-supervised by APM and Jilin University. The corresponding authors are Professor XU Jun of APM and Professor YU Jihong of Jilin University.
This research was supported by fundings from the Ministry of Science and Technology, the National Natural Science Foundation of China (NSFC), and the Chinese Academy of Sciences (CAS).
Link to the article: https://doi.org/10.1021/jacs.5c01756
