Recently, the Solid-State NMR and Heterogeneous Catalysis Team at the Innovation Academy for Precision Measurement Science and Technology (APM) has made progress in elucidating the mechanism of methanol-to-hydrocarbons (MTH) conversion over zeolite molecular sieves. The team discovered that Brønsted acid sites in molecular sieves can catalyze the Meerwein-Ponndorf-Verley (MPV) reduction reaction, which converts aldehydes/ketones into olefins during the MTH process. They also unveiled the role of aldehydes/ketones in the MTH reaction, and the related research findings have been published in Nature Communications.
The MTH reaction is a crucial chemical process that converts abundant coal, natural gas, biomass, and other carbon resources into fuels (such as gasoline) and chemicals (like light olefins and aromatics) through the methanol platform molecule. Understanding the MTH reaction mechanism is fundamental for developing high-performance catalysts and optimizing reaction processes. However, the intricate reaction network of MTH poses significant challenges in elucidating its mechanism, hindering the development of efficient catalysts. Over the past 40 years, significant attention has been paid to the direct reaction mechanism (forming the first carbon-carbon bond) and the indirect reaction mechanism (commonly known as the "hydrocarbon pool mechanism," which is the primary pathway in steady-state MTH reactions). Recently, it has been recognized that oxygenated organics, including acids/esters, aldehydes/ketones, are ubiquitous in MTH reactions. Nevertheless, the mechanism of aldehydes/ketones, particularly their role in MTH reactions, remains elusive.
Using in situ and two-dimensional solid-state NMR combined with isotope labeling experiments, the research team investigated the conversion of aldehydes/ketones in the MTH reaction. They found that aldehydes/ketones can undergo MPV reduction reactions with methanol molecules at Brønsted acid sites on molecular sieves. The team first employed two-dimensional 13C-13C and 13C-{1H} correlation solid-state NMR spectra based on dipolar coupling to structurally identify the surface-adsorbed species from co-reactions of acetaldehyde and methanol (Figure 1a), revealing the formation of various surface species. Then, they employed time-resolved in situ 13C solid-state NMR techniques to investigate the reactivity of these surface species (Figure 1b). Results showed that surface ethoxy species, formed through the MPV reduction reaction between acetaldehyde and methanol, emerged early in the reaction (at 8s) and served as crucial intermediates for ethylene production. As the reaction progressed, these surface ethoxy species gradually disappeared, indicating their high reactivity. Meanwhile, aromatic species and cyclopentadienyl cations emerged, which can act as "hydrocarbon pool" species and trigger indirect reaction mechanisms to form products. Chromatographic analysis of effluent products (Figure 1c) revealed that ethylene exhibited high selectivity early in the reaction, suggesting that MPV reduction was the primary pathway for early product formation. As the reaction progressed, ethylene selectivity decreased, and long-chain hydrocarbons emerged, indicating that indirect reaction mechanisms dominated later in the reaction. Since acetaldehyde species are often formed through direct mechanisms early in MTH reactions, its MPV reduction reaction with methanol can bridge the direct and indirect mechanisms of MTH reactions. Based on the experimental results, the research team elucidated the MPV reduction reaction mechanism of acetaldehyde and methanol on molecular sieves (Figure 1d) and correlated it with the formation of "hydrocarbon pool" species. In addition to acetaldehyde, the team discovered that acetone and other species can also undergo MPV reduction reactions with methanol at Brønsted acid sites, forming propylene. This research work sheds light on the role of oxygenated organics in MTH reactions and provides an important theoretical basis for understanding the MTH reaction mechanism.
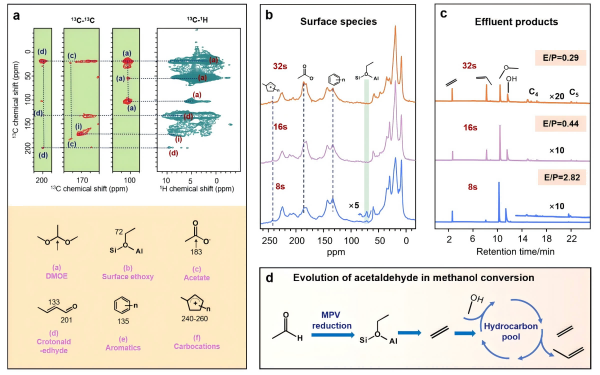
Figure (a) displays the two-dimensional 13C-13C and 13C-{1H} correlation solid-state NMR spectra of surface-adsorbed species resulting from the co-reaction of acetaldehyde and methanol on ZSM-5 zeolite; Figure (b) presents the in-situ 13C solid-state NMR spectra of surface-adsorbed species at various reaction times during the co-reaction of acetaldehyde and methanol on ZSM-5, along with the chromatographic analysis of effluent products (c); Lastly, Figure (d) explores the mechanism of theMPV reaction between acetaldehyde and methanol and its relationship to the formation of 'hydrocarbon pools'
The research was published in Nature Communications under the title “Unveiling the Brønsted Acid Mechanism for Meerwein–Ponndorf–Verley Reduction in Methanol Conversion over ZSM-5.” CAI Wenjin, a Ph.D. candidate at APM, is the first author of the paper, and Associate Researcher WANG Chao and Researcher XU Jun are the corresponding authors.
This research work was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology, the Chinese Academy of Sciences, and the Department of Science and Technology of Hubei Province.
Link to the article:https://https://www.nature.com/articles/s41467-024-52999-8
