Recently, the Biomolecular Dynamics Team from the Innovation Academy for Precision Measurement Science and Technology (APM), in collaboration with the Spectral Analysis Team for Complex Biological Systems, has made significant progress in the research on the dynamic activation mechanism of G-Protein-Coupled Receptors (GPCRs). The research team integrated all-atom molecular dynamics simulations and NMR technology to comprehensively decipher the dynamic process of the muscarinic acetylcholine receptor (M2R) transitioning from its inactive state to its fully activated state, and revealed the pivotal role of aromatic ring dynamics in the activation process of GPCRs. The relevant research findings were recently published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
GPCRs, as the largest family of membrane protein receptors in the human body, are the targets of over 30% of marketed drugs and more than half of drugs under development. Despite the fact that structural biology techniques such as cryo-electron microscopy have resolved numerous GPCR structures in recent years, several key scientific questions remain unresolved. These include how GPCRs achieve signal transduction through dynamic conformational changes, especially how various agonists precisely regulate the dynamic conformational changes of receptors, and the molecular mechanisms underlying the differences in efficacy among different ligands.
The research team employed molecular dynamics simulation techniques to conduct a systematic study of M2R in different functional states, including agonist/inverse agonist binding, nanobody binding, and G-protein binding. Through cumulative simulation data exceeding 100 microseconds, they captured two crucial intermediate states during receptor activation: first, the ligand-binding pocket contracts after agonist binding; second, dynamic changes and propagation in aromatic rings trigger partial opening of the TM6 helix. The research team discovered that the dynamic behavior of two key aromatic amino acids, W4006.48 and F3966.44, constitutes the "molecular switch" for signal transduction: when W400 binds to an agonist, it forms a stable stacking interaction through sidechain rotation, while the enhanced dynamic fluctuations of F396 facilitate the transition from the intermediate state to the activated state. The dynamic behavior of M2R when binding to different ligands also perfectly explains the chemical shift changes observed in the peaks of liquid nuclear magnetic resonance (NMR) experiments.
This research comprehensively reveals the temporal/dynamic process of receptor conformational changes, breaking through the theoretical framework of the traditional "two-state model (inactive/activated)" and demonstrating the important characteristic of multi-step dynamic pathways in M2R activation. More importantly, it discovers that the binding of different ligands is encoded and stored in the form of dynamic changes in the movements of aromatic rings. This finding provides a novel perspective for kinetic-based targeted drug design.
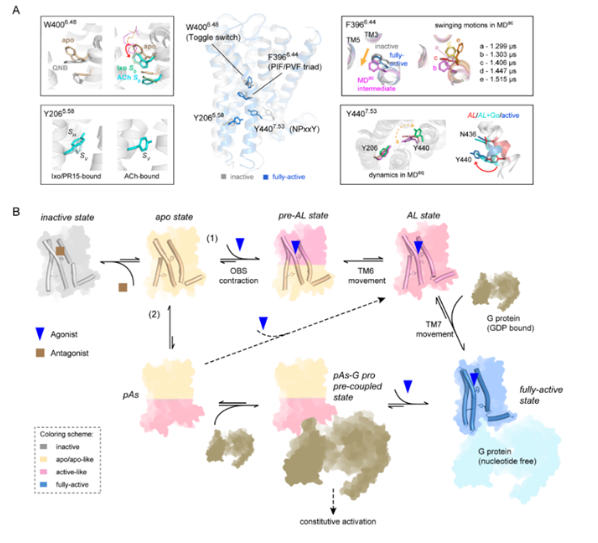
Schematic Diagram of the Activation Mechanism of M2R
The related research, titled "Visualizing agonist-induced M2 receptor activation regulated by aromatic ring dynamics," has been published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS). GONG Zhou, an associate researcher from APM, is the first and corresponding author of the article, while researcher HU Yunfei serves as a co-corresponding author.
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, the Wuhan Huanghe Elite Program, and other projects.
Link to the article: https://www.pnas.org/doi/10.1073/pnas.2418559122
