Recently, a research team from the Innovation Academy for Precision Measurement Science and Technology (APM) has achieved a breakthrough in dual-modal molecular imaging. By employing a relaxation modulation strategy, they have developed a highly sensitive 19F magnetic resonance-fluorescence imaging (19F MRI-FLI) agent. This work not only enables acid-base responsive 19F MRI-FLI at micromolar concentrations, but also provides a novel strategy for designing high-performance, responsive dual imaging agents. The findings were published in the Journal of the American Chemical Society.
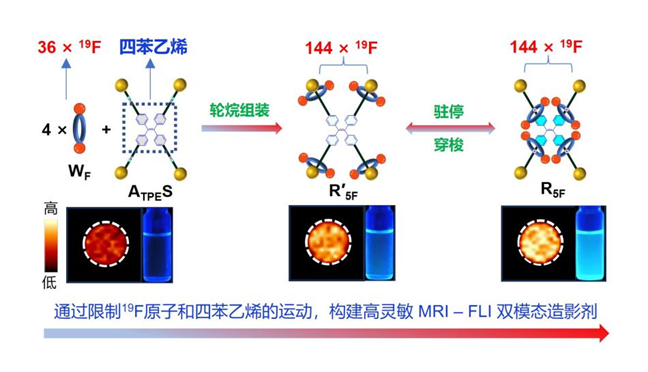
Responsive 19F MRI-FLI imaging agent based on fluorinated tetraphenylethylene rotaxanes
Dual-modal imaging integrates complementary techniques for precise and comprehensive imaging. For instance, FLI ensures high sensitivity for cells and superficial tissues, while 19F MRI provides quantitative "hotspot" imaging of deep tissues. Dual-modal FLI-19F MRI spans cellular to whole-animal imaging, making it widely applicable in disease mechanism studies, drug tracking, and therapeutic monitoring. However, integrating millimolar-sensitivity 19F MRI and micromolar-sensitivity FLI into a single molecule remains challenging.
To address the low sensitivity of 19F MRI, the team utilized a mechanically interlocking strategy using rotaxanes to efficiently and symmetrically integrate four fluorinated crown ethers (wheels), constructing a highly intense 19F MRI signal source composed of 144 magnetically equivalent fluorine atoms. Furthermore, the interlocked structure enabled the regulation of fluorine relaxation times. These strategies effectively bridged the sensitivity gap between 19F MRI and FLI, achieving dual-modal imaging at micromolar concentrations. Additionally, by leveraging the acid/base-responsive “stationing-shuttling” behavior of the wheels, TPE motions can be controlled, enabling stimulus-responsive FLI with “dim-bright” contrast.
By systematically comparing the changes in NMR and fluorescence signals, as well as conducting molecular dynamics simulations, the research team found that in the structure of rotaxane R5F, the four fluorinated crown ethers (wheels) are threaded onto the tetraphenylethylene core (axle) in a "C"-shaped conformation. Hydrogen bonding, steric hindrance effects, π-π interactions, and fluorous effects collectively restrict the motion of fluorine atoms and tetraphenylethylene, significantly enhancing both 19F MRI and FLI signals. This design enabled sensitive 19F MRI and pH-responsive FLI at a low molecular concentration of 16 μM. This study not only developed the most sensitive 19F MRI-FLI dual-modal imaging agent to date but also provided new insights into the kinetic behavior and structural design of rotaxane-based molecular machines.
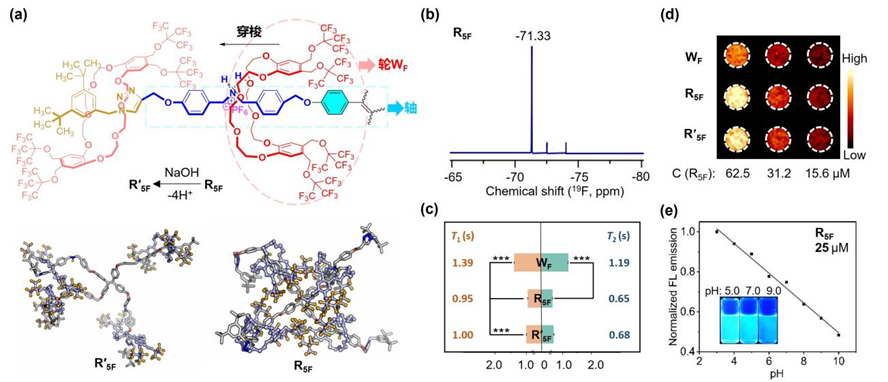
(a) Conformational changes resulting from deprotonation in rotaxane; (b) 19F NMR of R5F; (c) 19F relaxation times (T1 and T2) of Wheel WF, [5]R5F rotaxane, and R'5F, along with corresponding 19F MRI (d); and (e) pH-responsive FLI of R5F.
The research findings were published in the Journal of the American Chemical Society, under the title "Mechanical Interlocking of 144 Symmetrical 19F and Tetraphenylethylene for Magnetic Resonance-Fluorescence Dual Imaging." Doctoral student YANG Lan, postdoctoral researcher WANG Fang , and associate researcher LI Yu from APM are the co-first authors, with researchers JIANG Zhong-Xing and CHEN Shizhen as the corresponding authors.
This work was supported by grants from the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the Youth Cross-Disciplinary Team Program of the Chinese Academy of Sciences.
Link to the article: https://doi.org/10.1021/jacs.5c00429
