Recently, the ultra-sensitive magnetic resonance research team at the Innovation Academy for Precision Measurement Science and Technology has developed a novel "color-magnetic" encoding and labeling method. This method can be applied to ultra-sensitive "color" magnetic resonance imaging (MRI) of the lungs in vivo. The relevant research findings have been published in Nature Communications.
There is a wide variety of fluorescent molecules, which play crucial roles in fields such as protein labeling, fluorescence tracing, and immunofluorescence. Based on the differences in the emission wavelengths of fluorescent molecules, different substrates can be labeled with distinct colors, thereby enabling the simultaneous detection of multiple substrates. After functional modification, it can be employed for multi-target color-coded imaging of cells and tissues. However, the tissue penetration depth of fluorescence is limited, which poses limitations in in vivo analysis. Magnetic resonance imaging (MRI) has no penetration depth limitation and is widely applied in clinical disease detection. Chemical shift, as a crucial magnetic resonance parameter, plays a significant role in molecular structure identification and substrate detection. Similar to how different emission wavelengths of fluorescent labeling molecules correspond to distinct fluorescence colors, by constructing magnetic resonance molecular probes as "color-magnetic" labeling molecules, the measured targets can be "color-magnetically" encoded based on their different chemical shifts. This enables in vivo "color" magnetic resonance imaging (MRI) analysis of different targets. However, traditional proton probes have a narrow range of chemical shift variations, suffer from background signal interference, and exhibit relatively low detection sensitivity.
The ultra-sensitive magnetic resonance research team led by researcher ZHOU Xin has developed hyperpolarization technology, which can enhance the magnetic resonance signal of the inert gas ¹²⁹Xe by more than 100,000 times. The globally first self-developed "Human Lung Gas MRI System" has been granted the registration certificate for Class III innovative medical devices by the state. It has achieved simultaneous "structure + function coupling imaging" of the human body. This system has been widely applied in clinical settings in more than ten top-tier hospitals across the country, providing globally leading technology for the non-invasive assessment of major lung diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer. ¹²⁹Xe is sensitive to the chemical environment, exhibits a large range of chemical shift variations, and is thus well-suited to serve as a chemical shift probe for analytical detection. After inhaling hyperpolarized ¹²⁹Xe gas into the lungs and undergoing gas-blood exchange, the gaseous ¹²⁹Xe (¹²⁹Xe@gas), the dissolved ¹²⁹Xe in lung tissue (¹²⁹Xe@TP), and the bound ¹²⁹Xe interacting with hemoglobin (¹²⁹Xe@RBC) will exhibit different chemical shifts. Moreover, when ¹²⁹Xe binds to the functionalized "molecular cage" serving as a "color-magnetic" labeling molecule, a new specific chemical shift of ¹²⁹Xe within the "cage" can be generated. By employing MRI techniques to perform "color-magnetic" encoding on different chemical shifts, "color" MRI of multiple distinct targets in the lungs can be achieved. This provides an in-vivo non-invasive pathological detection method for the precise classification of lung diseases. However, the previously developed ¹²⁹Xe signals within the "molecular cage" were weak, making it difficult to simultaneously label signals with different chemical shifts in in-vivo applications. This has greatly restricted the application of molecular imaging based on hyperpolarized ¹²⁹Xe chemical shift encoding in in-vivo settings. Therefore, constructing "molecular cages" capable of binding hyperpolarized ¹²⁹Xe gas and enhancing the ¹²⁹Xe chemical shift signals within the "cages" is the key to achieving in-vivo "color" MRI.
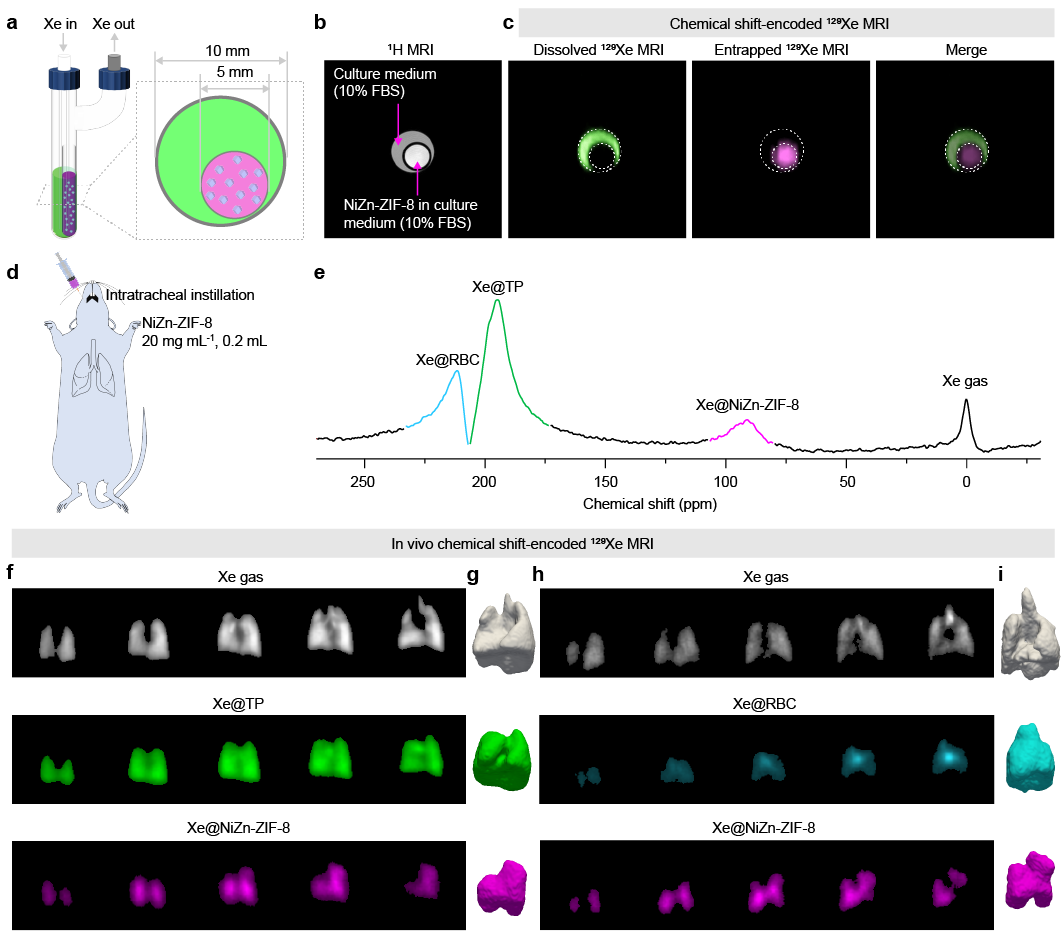
The multi-chemical shift imaging performance of the NiZn-ZIF-8 "color-magnetic" labeling molecule in both solution and in vivo environments
In living organisms, it is possible to clearly encode gaseous ¹²⁹Xe@gas, tissue-dissolved ¹²⁹Xe@TP, hemoglobin-bound ¹²⁹Xe@RBC, and caged ¹²⁹Xe@NiZn-ZIF-8, thereby achieving "color" MRI of the rat lungs in vivo
The research team led by Zhou Xin previously discovered that the metal-organic framework (MOF) material ZIF-8 has a high affinity for Xe and can be used to capture Xe atoms for hyperpolarized ¹²⁹Xe magnetic resonance imaging (PNAS, 2020). In this study, based on the foundation of previous research, the team introduced the multi-component MOF construction strategy into the field of hyperpolarized ¹²⁹Xe magnetic resonance for the first time. By introducing multiple metals into the ZIF-8 framework, the interaction between the ZIF-8 pores and Xe can be regulated, effectively enhancing the signal intensity of ¹²⁹Xe within the MOF cage in aqueous solution. The signal within the pores of NiZn-ZIF-8 constructed using this strategy is enhanced by more than 210-fold compared to the dissolved-state ¹²⁹Xe signal. When injected into the rat lungs as a "color-magnetic" labeling molecule, a strong signal of ¹²⁹Xe within the NiZn-ZIF-8 "cage" (¹²⁹Xe@NiZn-ZIF-8) can be obtained. Encoding and labeling four types of ¹²⁹Xe with different chemical shifts in the in-vivo lungs of rats (¹²⁹Xe@gas, ¹²⁹Xe@TP, ¹²⁹Xe@RBC, and ¹²⁹Xe@NiZn-ZIF-8, as shown in Figure 1) enables the acquisition of ¹²⁹Xe images in four different chemical environments. Moreover, the signals of ¹²⁹Xe with different chemical shifts can be effectively distinguished without mutual interference, that is, in-vivo multi-chemical-shift-encoded "color" MRI has been achieved. This MOF "color-magnetic" labeling molecule shares similar functions with fluorescent labeling molecules. Subsequently, functional modifications can be made to it according to specific requirements, enabling its application in ultra-sensitive color MRI for multiple biological targets.
The relevant research, titled "Multivariate metal-organic frameworks enable chemical shift-encoded MRI with femtomolar sensitivity for biological systems", was published in Nature Communications. Associate researcher ZENG Qingbin from APM and Ph.D. student YUE Quer served as co-first authors, while researchers ZHOU Xin and GUO Xiani acted as co-corresponding authors.
This research was supported by projects from the Ministry of Science and Technology of China, the National Natural Science Foundation of China, and the Chinese Academy of Sciences, among others.
Link to the article: https://www.nature.com/articles/s41467-025-62110-4
